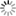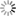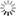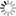Quantum Physics and Quantum Mechanics
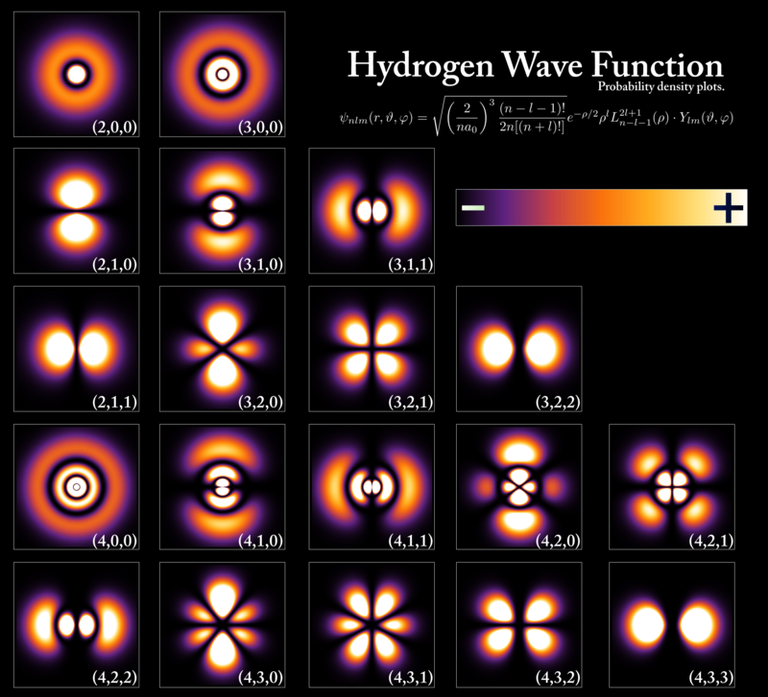
- [Hydrogen Wave Function - Wikipedia. (Wavefunctions of the electron in a hydrogen atom at different energy levels. Quantum mechanics cannot predict the exact location of a particle in space, only the probability of finding it at different locations.[1] The brighter areas represent a higher probability of finding the electron.)]
- Quantum Physics
What is quantum physics? Put simply, it’s the physics that explains how everything works: the best description we have of the nature of the particles that make up matter and the forces with which they interact.
Quantum physics underlies how atoms work, and so why chemistry and biology work as they do. You, me and the gatepost – at some level at least, we’re all dancing to the quantum tune. If you want to explain how electrons move through a computer chip, how photons of light get turned to electrical current in a solar panel or amplify themselves in a laser, or even just how the sun keeps burning, you’ll need to use quantum physics.
At a basic level, quantum physics predicts very strange things about how matter works that are completely at odds with how things seem to work in the real world. Quantum particles can behave like particles, located in a single place; or they can act like waves, distributed all over space or in several places at once.
In physics, a quantum (plural quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a physical property can be "quantized" is referred to as "the hypothesis of quantization". This means that the magnitude of the physical property can take on only discrete values consisting of integer multiples of one quantum.
- Quantum Field Theories
To begin with, there’s quantum mechanics, the basic mathematical framework that underpins it all, which was first developed in the 1920s by Niels Bohr, Werner Heisenberg, Erwin Schrödinger and others. It characterises simple things such as how the position or momentum of a single particle or group of few particles changes over time.
To understand how things work in the real world, quantum mechanics must be combined with other elements of physics – principally, Albert Einstein’s special theory of relativity, which explains what happens when things move very fast – to create what are known as quantum field theories.
Three different quantum field theories deal with three of the four fundamental forces by which matter interacts: electromagnetism, which explains how atoms hold together; the strong nuclear force, which explains the stability of the nucleus at the heart of the atom; and the weak nuclear force, which explains why some atoms undergo radioactive decay.
Over the past five decades or so these three theories have been brought together in a ramshackle coalition known as the “standard model” of particle physics. For all the impression that this model is slightly held together with sticky tape, it is the most accurately tested picture of matter’s basic working that’s ever been devised. Its crowning glory came in 2012 with the discovery of the Higgs boson, the particle that gives all other fundamental particles their mass, whose existence was predicted on the basis of quantum field theories as far back as 1964.
- Quantum Mechanics
The theory of quantum mechanics helps explain the natural properties of matter and light on atomic and subatomic scales, and serves as the basis for technologies such as lasers and semiconductor-based electronics. A second wave of innovation and discovery in the field is now underway, with new knowledge about quantum theory inspiring even broader applications of this research. One of the driving forces behind the second wave is a surprising convergence of physics, electrical engineering, computer science and mathematics.
Quantum mechanics is the branch of physics relating to the very small. It results in what may appear to be some very strange conclusions about the physical world. At the scale of atoms and electrons, many of the equations of classical mechanics, which describe how things move at everyday sizes and speeds, cease to be useful. In classical mechanics, objects exist in a specific place at a specific time. However, in quantum mechanics, objects instead exist in a haze of probability; they have a certain chance of being at point A, another chance of being at point B and so on.
Quantum mechanics has had enormous success in explaining many of the features of our universe. Quantum mechanics is often the only theory that can reveal the individual behaviors of the subatomic particles that make up all forms of matter (electrons, protons, neutrons, photons, and others). Quantum mechanics has strongly influenced string theories, candidates for a Theory of Everything.
Quantum mechanics is also critically important for understanding how individual atoms are joined by covalent bonds to form molecules. The application of quantum mechanics to chemistry is known as quantum chemistry. Quantum mechanics can also provide quantitative insight into ionic and covalent bonding processes by explicitly showing which molecules are energetically favorable to which others and the magnitudes of the energies involved. Furthermore, most of the calculations performed in modern computational chemistry rely on quantum mechanics.
- Three Revolutionary Principles
Quantum mechanics (QM) developed over many decades, beginning as a set of controversial mathematical explanations of experiments that the math of classical mechanics could not explain. It began at the turn of the 20th century, around the same time that Albert Einstein published his theory of relativity, a separate mathematical revolution in physics that describes the motion of things at high speeds. Unlike relativity, however, the origins of QM cannot be attributed to any one scientist. Rather, multiple scientists contributed to a foundation of three revolutionary principles that gradually gained acceptance and experimental verification between 1900 and 1930. They are:
- Quantized properties: Certain properties, such as position, speed and color, can sometimes only occur in specific, set amounts, much like a dial that "clicks" from number to number. This challenged a fundamental assumption of classical mechanics, which said that such properties should exist on a smooth, continuous spectrum. To describe the idea that some properties "clicked" like a dial with specific settings, scientists coined the word "quantized."
- Particles of light: Light can sometimes behave as a particle. This was initially met with harsh criticism, as it ran contrary to 200 years of experiments showing that light behaved as a wave; much like ripples on the surface of a calm lake. Light behaves similarly in that it bounces off walls and bends around corners, and that the crests and troughs of the wave can add up or cancel out. Added wave crests result in brighter light, while waves that cancel out produce darkness. A light source can be thought of as a ball on a stick being rhythmically dipped in the center of a lake. The color emitted corresponds to the distance between the crests, which is determined by the speed of the ball's rhythm.
- Waves of matter: Matter can also behave as a wave. This ran counter to the roughly 30 years of experiments showing that matter (such as electrons) exists as particles.
- Elementary Particles and Particle Physics
One of the primary goals in modern physics is to answer the question "What is the Universe made of?" Often that question reduces to "What is matter and what holds it together?" Elementary particles are the smallest known building blocks of the universe. They are thought to have no internal structure, meaning that researchers think about them as zero-dimensional points that take up no space. Electrons are probably the most familiar elementary particles, but the Standard Model of physics, which describes the interactions of particles and almost all forces, recognizes 10 total elementary particles.
Modern physics speaks of fundamental building blocks of Nature, where fundamental takes on a reductionist meaning of simple and structureless. Many of the particles we have discussed so far appear simple in their properties. All electrons have the exact same characteristics (mass, charge, etc.), so we call an electron fundamental because they are all non-unique.
The search for the origin of matter means the understanding of elementary particles. And with the advent of holism, the understanding of elementary particles requires an understanding of not only their characteristics, but how they interact and relate to other particles and forces of Nature, the field of physics called particle physics.
- Electrons and Related Particles
Electrons are the negatively charged components of atoms. While they are thought to be zero-dimensional point particles, electrons are surrounded by a cloud of other virtual particles constantly winking in and out of existence, that essentially act as part of the electron itself. Some theories have predicted that the electron has a slightly positive pole and a slightly negative pole, meaning that this cloud of virtual particles should therefore be a bit asymmetrical.
If this were the case, electrons might behave differently than their antimatter doubles, positrons, potentially explaining many mysteries about matter and antimatter. But physicists have repeatedly measured the shape of an electron and found it to be perfectly round to the best of their knowledge, leaving them without answers for antimatter's conundrums.
The electron has two heavier cousins, called the muon and the tau. Muons can be created when high-energy cosmic rays from outer space hit the top of Earth's atmosphere, generating a shower of exotic particles. Taus are even rarer and harder to produce, as they are more than 3,400 times heavier than electrons. Neutrinos, electrons, muons and taus make up a category of fundamental particles called leptons.
[More to come ...]